Arimistane on the Ropes after FDA Warning?
Mere weeks after the FDA unleashed an artillery strike against powdered caffeine, it has struck again. Upon inspecting VMI Sports' New York facility, the FDA sent the company a warning letter after finding "numerous" infractions.[1] Chief amongst these infractions was the manufacturing and sales of arimistane, an aromatase inhibitor, in several products sold by VMI Sports, formally known as Performance Nutrition Formulators LLC.

Arimistane FDA Warning: VMI Sports received a warning letter regarding Arimistane... but there's a whole lot more than meets the eye here.
For those unfamiliar, several warning letters have commonly foreshadowed previous ingredient takedowns, and this is typically the FDA's way of "soft removing" an ingredient from the market -- or at least beginning the official removal process -- but we're not certain that's the situation in this case.
We've yet to hear comment from VMI Sports, but as of right now, any products with this ingredient have been reformulated and/or removed from their website.
The FDA's new stance on Arimistane? Maybe sometimes...
The FDA attacked arimistane (also known as "Androsta-3,5-Diene-7,17-Dione", "3-deoxy-7-oxo-DHEA", or sometimes "3,7-keto DHEA") by claiming that VMI Sports was perpetuating false claims about the health benefits of the ingredient. They even classified arimistane as a "new drug". They cited section "201(p) of the FD&C Act"[1] as evidence for this claim.

Was it the product... or was it the claims made?! Either way, this product's going away, so see our VMI Sports Arimistane before it's gone
Summarizing the stipulations of this section is simple: as marketed, arimistane is in the same category as a prescription drug as the side effects can cause severe health risks like "decreased rate of bone maturation and growth, decreased sperm production, infertility, aggressive behavior, adrenal insufficiency, kidney failure, and liver dysfunction". The agency claimed it severely restricts other potent aromatase inhibitors for their potential to put patients at risk.[1]
Specifically, the products named "VMI Sports Arimistane" (now discontinued and out of stock at all PricePlow stores) and "A-XR PCT" caught the initial attention of the FDA. However, the warnings regarding the labels of VMI's "Cycle Guard" and "Vasogen" (neither of which contain the ingredient arimistane) tell a more complete tale of this action's significance, as the FDA deems them "unapproved new prescription drugs" and not "dietary supplements" in the letter.[1]
The FDA then demanded the removal of products containing arimistane from the VMI Sports line.
The FDA also adds their typical stance that,
To be a dietary supplement, a product must, among other things, "bear or contain one or more dietary ingredients' as defined in section 201(ff)(1) of the FD&C Act [21 U.S.C.§ 321(ff)(1))].
Section 201(ff)(1) defines "dietary ingredient" as a
- vitamin;
- mineral;
- amino acid;
- herb or other botanical;
- dietary substance for use by man to supplement the diet by increasing the total dietary intake; or
- a concentrate, metabolite, constituent, extract or combination of any dietary ingredient from the preceding categories.[1]
Emphasis ours above, which we'll discuss in the compliance discussion below. The FDA's enforcement team doesn't fully believe arimistane falls under any of those categories, or at least in this specific case, they don't consider it relevant.
Before going further, let's quickly talk about the ingredient itself, but first, you can also sign up for more Arimistane news from PricePlow in case we have to update this article:
What is Arimistane?
Arimistane, the ingredient in question, is a potent inhibitor of the enzyme aromatase. Aromatase is the enzyme that allows the body to form estrogen, as it converts androgens like testosterone into estrogen. AIs, or Aromatase Inhibitors, are often included in post-cycle therapy (PCT) regimen, as estrogen typically rebounds to sky-high levels after getting off of an exogenous anabolic cycle (this can often be noticed with several noteworthy YouTubers), and it's wise to keep estrogen low while your natural testosterone production rebounds.
While PCT is beyond this article, extreme cycles often require extreme -- or presecription-grade -- anti-estrogens. For the record, we haven't yet seen evidence showing how well arimistane works on its own in such situations, so it's not typically advised as the only anti-estrogen remedy after a "hardcore" cycle.
Anti-Fat? Not always...
For the time being, we believe arimistane is DSHEA compliant so long as your bottle and website are also compliant.
Many believe estrogen levels are correlated with less-than-ideal body composition, so they supplement with an aromatase inhibitor to keep body fat levels down and muscle mass high. Interestingly enough, this assumption is shut down by science. An investigation that tried to link body fat percentage to plasma estrogen found a nonlinear relationship. Levels of estrogen were lower in both high and low fat participants,[2] meaning diet and training likely had much more to do with it.
Either way, those are the two reasons arimistane is sold, and both have to do with reducing estrogen levels.
Now let's get back to the meat of the warning letter:
Prescription Drug Claims Made?
Another basis for the warning letter seems to be the claims that were made on the label and the VMI's website, such as,
- "Arimistane is a revolutionary Aromatase Inhibitor (AI) that effectively manages estrogen, and thus potentially vastly improves muscle energy, strength, mood & libido!"
- "Pure AI Modulator"
- "FIGHTING THE UPHILL BATTLE AGAINST THE AROMATASE ENZYME"
The FDA considers claims such as those to be drug claims (regarding products that are intended to "affect the structure or function of the body are defined as drugs").

Been a month or two since we've had to post this image. Last article was regarding concentrated caffeine being banned... yet the FDA not defining what
They also argued against the claims made on each ingredient, such as milk thistle and kudzu in Cycle Guard, and hawthorn in Vasogen.
These "prescription drug claims" seem to be the strongest part of the FDA's warning, as we discuss more about the potential legality of the compound later in this article.
Additional cGMP Violations
On top of the products / ingredients discussed above, the FDA's inspection found failure in certain manufacturing practices as required by 21 CFR 111, but it's our take that this letter is primarily due to the inclusion of arimistane and the claims made, and these manufacturing infractions were simply additional findings.
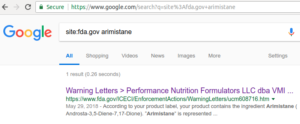
Per a basic Google search for site:fda.gov arimistane, this is the first time they've mentioned the ingredient on the entire site (as of June 6, 2018)
More to come against arimistane?
This is the FDA's first such time mentioning the ingredient on their website (see associated Google Search image), so it could have major implications on other brands.
Nobody knows if there's more to come, but you can probably guess the name of one brand that may indeed be willing to fight it:
Is arimistane compliant due to being a DHEA metabolite?
The legal compliance situation gets sticky when you see that arimistane is very similar to a legal ingredient, 7-Keto DHEA. 7-Keto DHEA has a legal place on the market, as GNC and Humanetics successfully received approval back in 1997.[3]
However, note that 7-Keto DHEA is not advertised as an aromatase inhibitor. Its function hardly resembles arimistane's! Slight structural differences like a methyl group here or an acetyl group there can entirely change the function of a hormone. Compounds with similarities can still have entirely different functions in the body.
While arimistane and 7-keto-DHEA may both be metabolites of the same parent molecule, DHEA, this does not mean they're similar compounds in terms of function.
Yet in terms of legality, functional and structural similarity don't matter as much as the word metabolite, which is where the argument heats up:
Hi-Tech Pharma's Legal Opinion
We contacted Jared Wheat of Hi-Tech Pharmaceuticals, and while we did not receive comment on the record, he was kind enough to send us a contracted legal opinion regarding DHEA metabolites that was published in 2015.
The paper was in regards to whether or not various DHEA metabolites are legal. This includes both the anabolic ones such as 1-Andro, but also Arimistane:[4]
DHEA is definitely a legal supplement. But what else is?
The story starts with DHEA, or Dehydroepiandrosterone. DHEA itself is legal, as it is grandfathered in as an "Old Dietary Supplement" due to its being on sale as a supplement prior to 1994. Further, DHEA is naturally produced, and is a metabolic precursor for several hormones such as testosterone and estrogen. Its clear basis in nature also solidifies its legal sale as a supplement.

Of course Hi-tech Pharma has a legal opinion on the matter that they'd be willing to share... and it points us to the citation we need!
Additionally, DHEA is specifically exempt from the Anabolic Steroid Control Act of 2004 and the Controlled Substances Act.[5]
So DHEA is legal. That much is never really argued.
Now let's get back to DSHEA 1994, the law our elected representatives in congress passed that defines a dietary ingredient as "a concentrate, metabolite, constituent, extract or combination of any dietary ingredient from the preceding categories". Emphasis on the word "metabolite" is ours once again:
So is arimistane a metabolite of 7-Keto DHEA?!
According to Hi-Tech's legal letter, which calls arimistane by its 3,7 Keto DHEA moniker, they claim that it is a naturally-occurring metabolite of DHEA, and is found in the human urine. They further claim that both 7-Keto DHEA and arimistane cannot convert to testosterone, estrogen, or progesterone, citing a modified image from a study to show the reaction.[6] This allegedly clears both ingredients from being banned by the Controlled Substances Act and Anabolic Steroid Control Act of 2004.
If the "naturally-occurring metabolite found in human urine" statement is indeed true, and no other medical claims which would qualify it as a drug are made, they consider it legal as a dietary supplement under DSHEA. This can all be found towards the end of Hi-Tech's legal opinion.
But the above bolded comment regarding medical claims brings us back full circle to the prescription drug claims discussed above.
FDA doesn't care because of the claims and "safety issues"?
Everything above from Hi-Tech's legal opinion is well and good, but the FDA isn't necessarily arguing that this isn't a metabolite! And they may not need to in this case!
Instead, the FDA's pushing that it is now a prescription drug due to "its toxicity or other potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its use, it is not safe for use except under the supervision of a practitioner licensed by law to administer it", on top of the structure claims.
So on top of everything else, the legality of the substance is also being questioned for safety reasons, not necessarily structural similarity. Remember that the burden is on the FDA that it needs to bring proof that a compound is not safe, which is another battle in its own, but the feds have now thrown in three arguments against VMI.
Now what? Is arimistane dead?
We'll have to see if this evolves into a court battle, but our guess is this: VMI seems to have removed the ingredient, and will probably pay the piper, tone down their verbiage, and move forward with their successful products such as the high-energy K-XR pre workout.
But if this letter were to come to Hi-Tech Pharma, they would likely bring the fight, citing the aforementioned legalities as a metabolite. They just better be damn sure not to make anything even remotely sounding like a prescription drug claim.
Make sure to stay tuned for our constant updates on the situation, but for the time being, we believe arimistane is compliant so long as your bottle and website are also compliant.
Lessons learned?
With as many regulations that are out there, playing in the post-DASCA game of selling hormonal supplements is a risky endeavor. Companies need to be ridiculously careful regarding the ingredients used, and even if they're compliant, there are other ways to get hit.
We've always stood with 1994 DSHEA compliance as written by our elected representatives in congress. Arimistane seems to be compliant as a "metabolite" of the legal ingredient, DHEA. But that doesn't mean it's going to be universally legal in every bottle out there, because there are more laws than just 1994 DSHEA that a company needs to concern itself with.
Wishing the best for VMI

Don't let this article fool you - we are big fans of VMI Sports, and hope they clean up their site and labels and pound out more of that crazy K-XR pre workout! Just let this be a lesson learned for everyone.
We are also undoubtedly fans of VMI Sports and their owners, and we hope they survive this and come back stronger than ever. But it's without a doubt a lesson learned in FDA/FTC compliance. Just because your product is manufactured in a cGMP compliant facility doesn't mean that it's automatically legal - especially when you start getting overzealous on that label and website.
Our take? Brands best be careful
Your family at PricePlow are not huge fans of market regulation. If we could, we'd prefer a world where the consumer has the utmost freedom for using their Constitutionally-granted purchasing power. However, we do prefer our supplements to come without medical claims, as consumers should do the research themselves.
And in the case of arimistane, where research and even anecdotal data are sparse (at best), it may be worth saying nothing at all on those bottles.
Want to stay up to date on our Arimistane news? Sign up for Arimistane news updates using PricePlow below, or check out our VMI Sports page to compare prices:
Update: Bruce Kneller Disagrees. Arimistane may be in trouble
The following was emailed to us with a subject line of You are wrong:
It is not relevant whether androsta-3,5-diene-7,17-dione (to remove any doubt as to the moiety I refer to, I am referring to the moiety with a CAS# of 1420-49-1) is a metabolite of DHEA or 7-keto-DHEA or macaroni and cheese.
That does not make the moiety absolutely & automatically legal as a dietary ingredient.
It does make it "potentially" a dietary ingredient if the following are met:
- The moiety itself has been used as and sold as a dietary ingredient in a dietary supplement in the United States on/before October 15th 1994 or;
- The moiety itself as itself, is found naturally in a food stuff eaten in the United States of America (I do not believe that human urine is a food stuff but Mike Mentzer, may he rest in peace, might disagree with me) and;
- The moiety has not been "substantially investigated" for use as a drug (the geography not well defined by statute) before any of the above situations.
Additionally, if the moiety is such that typical usage to utilize the moiety in a "safe" manner requires such skill and education that a layperson can not be expected to do so (e.g., could only be reasonably expected to be safe under the direct supervision of a physician) then the moiety will very likely be considered not just a drug but a "prescription drug" which will mean it is not a dietary ingredient nor can it be labelled as such in a dietary supplement.
As someone who was directly involved with, singularly designed, and was the clear inventor of the undisputed best selling "post cycle therapy" moiety ever to be marketed as a dietary ingredient in the history of dietary ingredients to date in United States, that being androsta-1,4,6-triene-3,17-dione (also known as "ATD") of which eight (8) valid United States Patent claims were awarded to me by the USPTO (see US Patent #7,939,517) as an individual in 2011 and to which the FDA issued on March 2nd, 2011, to my then employer (Gaspari Nutrition), a Warning Letter quite similarly worded to the recent Warning Letter sent to VMI, specifically, with FDA commenting on the marketing and selling of any putative dietary ingredients/supplements making any claims to possessing or inducing anti-aromatase or estrogen reducing/blocking characteristics, I feel I am uniquely qualified to comment with great authority on this matter.
I interacted with FDA on almost a daily basis for approximately six (6) weeks between January and February of 2011 with a variety of government officials, ranging from the local, district inspectors all the way up to the Associate Commissioner of the FDA. In no uncertain terms I can write that based on my interactions with the FDA at that time, that the FDA probably feels that any/all moieties that make claim(s) to having anti-aromatase or estrogen lowering qualities and clearly are labeled to such effects are indeed, prescription drugs, are mis-branded and are thus, adulterated.
I have seen the letter that Mr. Wheat's legal team has issued and which Mr. Wheat has circulated long before it appeared on your website. While I am clearly not an attorney and my thoughts should never be construed as legal advice, I absolutely disagree with the conclusions in that letter. My thoughts are that anyone utilizing just that letter for "legal cover" might want to consider - at the very least - having other attorneys write concurrent opinion memoranda about this topic. I would suggest attorneys Rick Collins and/or Erica Stump for starters.
Clearly, we are going to see the number of "stuff" available to the general public as dietary supplements reduced substantially by FDA and other government agencies in the ensuing months/years.
This is unfortunate but is it the expected, natural by-product of a shoddy-written law (DSHEA 1994) which clearly needs to be discarded and re-written for clarity.
Regards,
-- Bruce Kneller
Thank you Bruce, as always.
As always, sign up for Arimistane news updates using PricePlow on this page, it seems like this may have heated up even more than we thought.
References
- Food and Drug Administration; "Warning Letters - Performance Nutrition Formulators LLC dba VMI Sports 5/18/18. (n.d.)"; May 18, 2018; https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm608716.htm
- Ziomkiewicz A, Ellison PT, Lipson SF, Thune I, Jasienska G. Body fat, energy balance and estradiol levels: a study based on hormonal profiles from complete menstrual cycles. Human reproduction (Oxford, England). 2008; 23(11):2555-63. https://pubmed.ncbi.nlm.nih.gov/18641044
- Center for Food Safety and Applied Nutrition. (n.d.). New Dietary Ingredients Notification Process - New Dietary Ingredients in Dietary Supplements - Background for Industry. Retrieved from https://www.fda.gov/Food/DietarySupplements/NewDietaryIngredientsNotificationProcess/ucm109764.htm
- DeLong Caldwell Bridgers & Fitzpatrick Law Firm; "RE: Request for Opinion"; November 16, 2015; https://blog.priceplow.com/wp-content/uploads/dhea-legal-opinion-hi-tech-pharmaceuticals.pdf
- One Hundred Eighth United States Congress; "S. 2195 (108th): Anabolic Steroid Control Act of 2004"; October 8, 2004; https://www.govtrack.us/congress/bills/108/s2195/text/enr
- Numazawa, Mitsuteru, et al; "Synthesis of Androst-5-en-ones and Androsta-3,5-dien-7-ones and Their Related 7-Deoxy Analogs as Conformational and Catalytic Probes for the Active Site of Aromatase"; J. Med Chem; 1994; 37, 2198-2205; https://pubs.acs.org/doi/abs/10.1021/jm00040a012 (backed up at https://docdro.id/Ga0NkL4)
![Image courtesy Hi-Tech Pharma, via legal opinion<sup>[6]</sup>](https://blog.priceplow.com/wp-content/uploads/dhea-7-keto-dhea-arimistane-625x255.png)





