November 2017 Update: The first SARMS warning letters from the FDA have gone out to three companies!
Prohormones (PHs) were a mainstay of the bodybuilding supplement industry since Patrick Arnold first introduced the world to 4-androstenedione around 1996 until the passing of the DASCA bill in December 2014, effectively banning prohormones once and for all. Since then, extreme muscle-building consumers and companies alike have been looking for alternatives – and many of them have turned to another set of aggressive compounds, SARMs.
SARMs are non-steroidal compounds that still activate the androgen receptors, and according to user feedback typically give similar results to a light PH or steroid cycle.
Up front, this all sounds good - the next PH replacement is here… right? But as you can imagine, it's not always that simple.
On one hand, consumers have made it extremely clear that they demand advanced muscle-building agents that they can discreetly purchase online, rather than deal with shady characters in the gym locker room. And a few of these compounds do indeed show serious promise, despite their still-limited research.
And on the other hand, it's tough to dispute the illegality of the production, sales, and distribution of most (if not all) of these compounds, and we ultimately see this ending with some kind of stain on our industry.
But that gets ahead of ourselves, so let's start with the consumer-facing section:
The History of SARMs
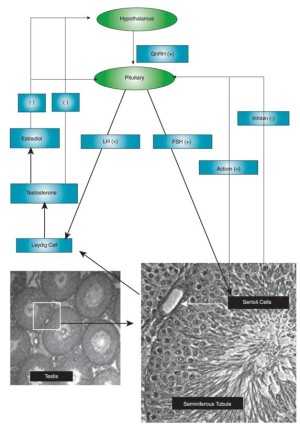
The introduction and discovery of SARMs into the scientific community was indeed a significant breakthrough to both medical and anti-aging organizations.[1] The most common problem with androgen therapy (ie HRT -- hormone replacement therapy) is the limited availability of safe compounds to administer, without some of the detrimental side effects that exogenous administration of natural androgens cause.
"The ultimate goal of research in this field is to discover chemical compounds that can be used for androgen replacement therapy to address one or some functions of prototypic steroidal androgens without unwanted side-effects."[2]
The effort of most SARM researchers is to acquire an agonist of the androgen receptor without the susceptibility of binding to and serving as substrates for aromatase and 5a-reductase (or possessing androgenic activity of its own). It was hoped that this could be achieved by either steroidal or nonsteroidal means, and efforts to synthesize compounds are still underway.
By big pharma, for big pharma
Note that this is all research done in the name of better prescription drugs, not research for the muscle-building industry. Several Big Pharma companies are doing the research, and their goals are typically a bit different than our average readers.[2] For instance, they want drugs that prevent muscle-wasting in the elderly and for post-surgical patients… whereas you just want to get swole. We talk about some of that research (and the patents) below.
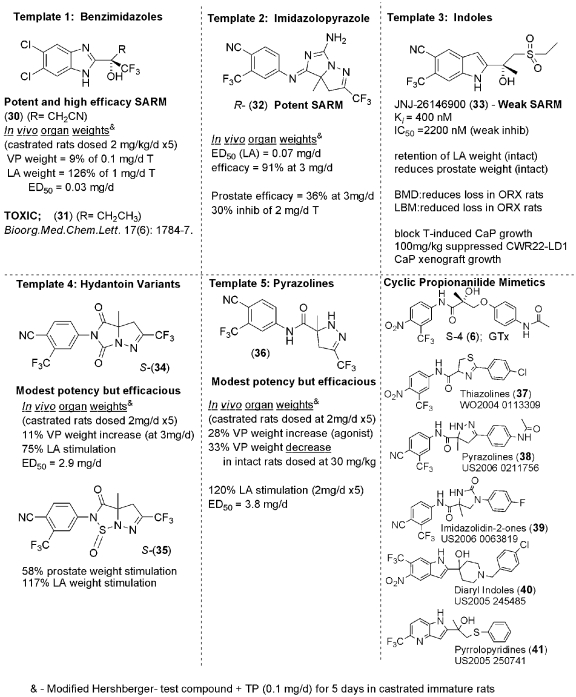
These compounds were developed and research was largely funded when it became apparent that chemical modifications to the antiandrogen bicalutamide forged androgens (ligands) with agonistic activity.[3] There are many different classes of SARMs and there have been extensive efforts over the years to synthesize and test them for favorable qualities.
Hypothalamus-Pituitary-Testis Axis Of Androgen Regulation
While it's still too early to officially say so, the good news is that the pharmaceutical companies seem to be succeeding in their early-scale efforts. Certain low-dose SARMs are showing to be effective in their case subjects (such as the elderly) with minimal side effects, which is more than you can say about a lot of PH or other designer drugs.
Variability in SARMs
What makes SARMs unique is the variability in structure among them. Technically, anything that binds to the androgen receptor and activates it in certain tissues more than others can be classified as a SARM, which means there is little to no structural basis behind this naming classification, unlike that seen of steroids (4-ring base).
Not a steroid
This is also what made it the "Next Big Thing™" after the PH ban - they are not "steroidal" in the technical sense, so the 2014 DASCA law's verbiage did not cover them.
People often confuse SARMs with steroids: a SARM can be a steroid and a steroid can be a SARM, but when we talk about SARMs on the market, what we're talking about is usually a Nonsteroidal Selective Androgen Receptor Modulator.
Let's break down what this means:
- Nonsteroidal meaning it does not have a 4-ring base structure or the steroid nucleus.
- Selective meaning it varies where it is active (tissues).
- The rest denotes ability to bind and activate or modulate the Androgen Receptor.
SARM Selectivity is Key
Tissue selectivity is imperative to the medical and therapeutic benefit of a selective androgen receptor modulator.[4] For a SARM to be considered medically useful, it must have favorable tissue selectivity, meaning very little interaction with secondary sex tissues (seminal vesicles and prostate) while demonstrating agonist activity in bone and muscle (gains).
"Side effects associated with classical anabolic steroid treatments have driven the quest for drugs that demonstrate improved therapeutic profiles."[4]
Basically, a good SARM is one that selectively causes muscle growth, yet leaves your liver, prostate, hairline, and boobs alone. It should also ideally possess anabolic activity similar to testosterone or be more potent than this natural hormone.
Each SARM requires its own research
Since these compounds are so variable, the muscle-building industry is really doing everyone a disservice when lumping them all together under one term. After all, each one may select something completely different, and have different effects on the body.
Because of that, you need to research each one on its own grounds. They're not all the same, and the word SARM is really pretty meaningless for your purposes.
It's not like protein, where we can get reasonably similar results between the different types within the category (ie whey, casein, and pea aren't that much different at the end of the day). But with the various SARMs finding their way onto the market, one may work far differently than another.
Point being, if Joe Bloggs at the gym says "SARMs are awesome, you should try them", and he doesn't tell you what exact SARM (or in what dose and what PCT he uses), then he's an idiot who hasn't done his research. Listening to people like this instead of doing your own research puts yourself at risk.
Not for drug-tested athletes… but they once were...
Note that SARMs are banned by WADA (the World Anti Doping Agency),[5] so if you're a drug-tested athlete, don't even think about it.
Interesting to note that certain SARMs were the doping agent du jour during the 2008 Beijing Olympics, according to certain sources.[6] Yet WADA has known about these since 2007, as reported by the MIT Technology Review,[7] and they were on the 2008 banned substances list[8] (whether they could actually detect all of them back then is another question).

Jose Canseco Mark McGwire - More than just Creatine Users. SARMs were clearly the 'dope dujour' back in cerca 2008, and postulated to be the 'smart' doping choice for the MLB around that time as well, yet WADA has long been on to the compounds. Due to timing, it's unlikely the two characters above ever touched them, but our faithful readers know that we love to use this picture any chance we get.
Jose Canseco Mark McGwire - More than just Creatine Users. SARMs were clearly the 'dope dujour' back in cerca 2008, and postulated to be the 'smart' doping choice for the MLB around that time as well, yet WADA has long been on to the compounds. Due to timing, it's unlikely the two characters above ever touched them, but our faithful readers know that we love to use this picture any chance we get.
SARMs were also hinted as the thing that the "smart" guys in the MLB were doing in early 2009.[9]
(Meanwhile, advanced dopers who get drug tested have likely moved on to something we've never heard of, and blogs like PricePlow will finally write about in 2020, but we digress…)
Anyway, the point is that the drug-testing world caught up with this long ago, but a key takeaway is that while this is "new" to our industry, they're not necessarily that new...
Investigated by Big Pharma
A number of synthetically derived SARMs have been investigated from various pharmaceutical companies such as Ligand Pharmaceuticals (LGD-4033),[10,11,12,13,14] Merck,[15] Johnson & Johnson,[16,17] GlasoSmithKline,[18], and GTx (Ostarine / MK-2866).[19,20]
The most novel compounds, also known as lead compounds, are SARMs that display preferential partial agonist activity in prostate and seminal vesicles, but which also display anabolic activity in bone and muscle tissue. GTx developed a series of SARMs with the most notable one being Ostarine / MK-2866,[21,22,23] which will be the focus of the next article in this series.
The good news is that these are legitimate companies putting a lot of time, money, and research into these drugs. Some of the research has been promising, heading into human trials, albeit at lower doses than are being sold by some underground "supplement" companies. The bad news is that they are just as described - investigational prescription drugs - which segues into some of the legal issues discussed in the next section below.
No statements on this page have been approved by the FDA. Supplements, dietary program changes, and anabolics can only be used with your physician's written consent.
We encourage you to continue reading, as the drama is just about to begin:
SARMs are not legal supplements
Update - October 21, 2015: The first SARM lawsuit has been filed! See our SARM Lawsuits page for more details.

The FDA is on it...
The FDA is on it...
Why are we here anyway?
Realize that after the DASCA bill was passed, several 'hardcore' companies had their lives put on the line. It was to either come up with the next thing, or go broke and out of business. Some companies got out of the gray area business once and for all, branching off into DSHEA compliant products. Yet others got into the business, clearly taking the legal risk for some quick profits.
They may not violate DASCA… but that doesn't mean they're legal
The reasons SARMs have been the Next Big Thing™ in terms of muscle-builders is because several of them have proven to work, and DASCA does not specifically include anabolic compounds with nonsteroidal structure. It doesn't currently specifically list any SARMs either. However, that doesn't mean they are legal to sell by any means.
At best, SARMs are "research chemicals": not completely illegal, since many are not prescription drugs or scheduled drugs (or analogs of such drugs).
But when they're being sold as supplements or at supplement stores, it is clearly a violation of the FDA's DSHEA law. This is the first avenue where companies and their leaders are taking on risk - if the FDA or DEA wants to get involved, they easily could.
Remember that you're watching a live game of cat and mouse here (and the cat's move is next).
But what is a supplement?
While writing this article, we reached out to a few SARM manufacturers, and a couple of them pointed out that this isn't the first time a non-supplement has made its way into supplement stores.
For instance, there's phenibut. This is also a Russian prescription drug, and is horribly addictive to top it off. It's sold in various "supplements" as well.
So your favorite SARM manufacture will happily argue that a nootropic drug, which works in the brain, is potentially more hazardous than a selective androgen receptor modulator that works in muscle tissue. Meanwhile, they'll argue that there's precedence for this kind of behavior, which is rarely being punished or enforced by the FDA.
But if it's not specifically illegal, does that mean it's legal?
UPDATE: One industry veteran's response
The day after posting this article, we received this email from an industry veteran:
Two comments:
- Noopept is probably DHSEA compliant. Noopept is a known PRECURSOR to an endogenous peptide found in most mammal species. It is also NOT considered a racetam. Check DSHEA about precursors.
- Phenibut is a drug, I agree.
The issue here is two wrongs do not make a right.
It's still illegal. 100% so.
Update 2: this goes even further. Click here to read Bruce Kneller's arguments regarding FDA enforcement and how SARMs should be classified as prescription drugs.
Comparing to the PH situation
SARM manufacturers also point out that PH's were practically illegally sold for over ten years (depending on how you look at the Anabolic Steroid Control Act and its updates in 2004[25]), and the various PHs and designer compounds seem to be far more dangerous than the most well-researched SARMs, given the available data.
And in the same breath of air, they'll remind you that several of your favorite above-board supplement companies were "financed" by selling these illicit "supplements". The cycle repeats.
"If it bleeds, it leads"
Where is this going?
The point is, non-DSHEA compliance doesn't seem to be a concern to many users or even several large companies. What does seem to be a bigger concern is the inherent danger, and SARM companies are quick to state that these aren't as dangerous as previous alternatives, given what they know so far. That leads into a point we make later on, where the media has been relatively uninvolved since "if it bleeds, it leads"... and nobody's child has ended up dead from this stuff (yet, at least).
Points all well-taken.
So where's the FDA at then?
FDA action so far
As of right now, only one warning letter has been sent one company (BioGenix), and the FDA's action was most likely primarily due to the fact that they put unlabeled Cialis and Levitra in the product, at a serious hazard to their customers.[26]
![Tadalafil and nitrates. you wouldn't think we'd need to ask this, but could you people *not* put boner medication into workout supplements, please? this is what happens to blood pressure when nitrates are added in.<sup><sup>[27]</sup></sup>](https://blog.priceplow.com/wp-content/uploads/tadalafil-nitrates.png)
Tadalafil and nitrates. you wouldn't think we'd need to ask this, but could you people *not* put boner medication into workout supplements, please? this is what happens to blood pressure when nitrates are added in.<sup>[27]</sup>
Tadalafil and Nitrates. You wouldn't think we'd need to ask this, but could you people *not* put boner medication into workout supplements, please? This is what happens to blood pressure when nitrates are added in.[27]
Research chemicals?
So what does "research chemical" mean?
Ostarine, for instance, is an investigational new prescription drug. A company named GTx has been working on whether this might have a chance at becoming a prescription drug.[15,19,20]
Yet, while it is technically their IP, it can obviously be found in various underground websites that sell such research chemicals. When these stores say that they are 'not for human consumption' or 'for research only', yet include information about oral dosing for humans… you can guess that it's only a matter of time until someone gets taken down.
We're not lawyers, but it seems like these practices give law enforcement all the grounds they need to go bashing doors in.
After all...
- Is it a drug? Yes.
- Are you the approved distributor of that drug? No.
- Is it patented? Oftentimes, yes.[28,29] (note that there are countless SARM patents)
- Are you introducing a counterfeit drug into interstate commerce? Yes.

We don't claim to be legal advisers or anything... but this is probably not a company whose IP you want to steal from
We don't claim to be legal advisers or anything... but this is probably not a company whose IP you want to steal from
Now, Ostarine has made it even further than those underground research chemical sites -- it's now plainly listed on several of the supplement stores that PricePlow works with, and rumor has it that a relatively large brick-and-mortar retailer will be carrying some SARMs soon enough as well.
The ultimate legality may come down to a compound-by-compound basis, but don't delude yourself into thinking this is legal (or not illegal). It might be "flying under the radar", but that is no indication of actual legality.
As if the DEA cared...
And even if you can argue some gray area technicality, do you think for one moment that the DEA even cares about legal technicalities? They'll raid your warehouse like a band of stormtroopers, take everything they see, and lock it into a vault while you go bankrupt. You didn't expect them to play fair, did you?
What will likely happen
So whose job is it to enforce the patents? There are several federal laws that protect patent holders,[30] but does GTx need to pursue these companies themselves, or does the government? They clearly have a case (someone else is making tons of money off of their research and IP, while they aren't), but do they care yet? Does the DEA? Where is the line drawn, and when?
Anyway, due to the fact that DASCA contains a provision that basically any compound can be added to the ban list, it's not hard to imagine that they might just add these compounds to the list and be done with it. That's probably where this chapter of the industry quietly ends, in our opinions at least.
But users don't care about legality, do they?

SARMs rumored to be coming to a mid-sized supplement store chain near you. Fitting image courtesy UsedToBeATacoBell.com
SARMs rumored to be coming to a mid-sized supplement store chain near you. Fitting image courtesy UsedToBeATacoBell.com
Nevertheless, various companies have taken to sell them both online and in small brick-and-mortar stores, despite their non-compliance, and users are buying them in droves -- without doing proper research.
The end-user lack of research is where we take issue, since these users all run the risk of potentially harming themselves in the process (such as by taking too much or not running PCT). Adding to the problem, the "supplement companies" selling these drugs don't seem to be educating their customers either.
For instance, several companies are advertising doses of Ostarine as 15mg-30mg, stating that you don't need to run post cycle therapy. However, the research-based dose of ostarine was at 3mg,[31][32] and it dropped test production by 25%![31] What in the hell do you think is going to happen when you run it at 10x that dose?!
Should you trust someone who's breaking the law at a profit?
The bigger issue from a consumer's point of view is, how do you trust someone who's openly violating the law -- and profiting off of that?
Some of the "supplement companies" have popped up from out of nowhere, here to make a quick buck. How can anyone be sure of what they're getting?
This isn't something found in nature (like the stimulant found in geranium flowers). This is flat out illegal and the companies selling them know it. What's stopping anyone from breaking the law further by missing 'label' claims? It's not like it would have been legal in the first place!
So if you're going to step into this fold, you better hope to death that you're getting what they're saying they're giving you, in the dose claimed. Third party lab tests -- not tests from the manufacturer or raw material suppliers -- are paramount. But do they exist?
The research is still a bit flimsy
Meanwhile, the fact is that nobody in the supplement industry has anything more than a surface level clue about these first/second generation SARMs. Big pharma might not know much more, and if they do, they currently have no obligation to share it with us.
Many of these are effectively failed prescription drugs whose research was aborted upon their failure. Still others have their research still in progress, but have yet to gain approval (the primary example being GTx and Ostarine).
GTx has even go so far to state on their SEC reports,
"We do not expect to obtain FDA or EMA approval, or any other regulatory approvals, to market any of our product candidates, including enobosarm (ostarine), for the foreseeable future, and it is possible that none of our product candidates will ever receive any regulatory approvals.[33] -- GTx Inc. SEC Report, June 30, 2014
Meanwhile, their quarterly loss was just under $48 million for 2Q 2015.[34] This does not paint a rosy picture in terms of approval or safety, and that's for the most well-researched SARM out there!!
The dangers of some compounds: Worth risking cancer?
Even worse, one compound named GW-501516 / Cardarine was abandoned for research because it caused cancer in animal studies at all doses, at all durations of use.[35,36,37,38]

GW-501516 research was discontinued because the lab rats kept getting cancer and dying (and John Coffee was no longer around to save them). But let's not let that stop us from selling them as supplements!!!
GW-501516 research was discontinued because the lab rats kept getting cancer and dying (and John Coffee was no longer around to save them). But let's not let that stop us from selling them as supplements!!!
Is anything worth that kind of risk?!
Arguments on GW-501516
It's worth noting that the response to these allegations is that it is generally unlikely that PPAR is going to stimulate cancer growth in human cell lines - a valid case - but again… is anything worth that risk?
It's also worth noting that GW-50516 is not even a SARM. However, it's classified as one by several websites that sell the stuff, which reaffirms our pleas for users to do their research, lest the blind lead the blind!
Have things really gone this far? Spending $55/month for something that probably increases some parameters of endurance exercise… that has literally never been given to a human in a clinical setting?
This is the kind of territory you're getting yourself into here, folks. No man's land. There's no reason to be someone else's petri dish.
This probably won't end well.
So why do we care? Because there's a chance that this ends up in USA Today and every other major newspaper, and the media is undoubtedly going to make all of us look like scum. Every last one of us, from consumers to brands to industry advocates, will end up far worse if that happens.
But so far, nobody seems to care much. MAXIM magazine already covered this months ago,[39] and apparently Marc Lobliner's been questioned by a massive men's print magazine as well. That's just the beginning… but so far, not much fanfare.
If it bleeds, it leads...
As the phrase goes in the media industry, "If it bleeds, it leads" - but nobody has gotten seriously hurt (yet).
These compounds - at least ostarine and andarine - do seem safer than most of their predecessor alternatives, specifically PHs. But that may not matter - all it will take is for one idiot to drink himself to death while he has some kind of SARM in his bloodstream, and you can imagine the media shitstorm that will come next. (This is also why we can't have nice things.)
So how can any level-headed company not expect this to end poorly for the industry?
At best, nobody is hurt, and some people have some gains. Eventually, DASCA may be revised to ban them, nice and quietly.
At worst, something stupid happens, which catalyzes the next John Oliver segment on the supplement industry and the next FDA push to stifle any room for legal innovation in the industry just because a few people were greedy. Conveniently during election season, no less.
It's one thing to have these compounds in obscure places that only well-researched steroid users know about. But selling this through popular brick and mortar stores as a dietary supplement is risky to the nth degree for everyone involved.
But in the meantime, they will sell, and they will sell well, so we just urge users to do their damned homework before proceeding. And that means to understand that SARMs aren't supplements, they are investigational drugs that should be treated with the care of steroids.
Good luck, folks. Stay safe and do your research.
Bruce Kneller's Opinion regarding FDA legality
This was emailed to us on September 1, 2015:
FDA and various US Attorneys may very likely be inclined to view all SARMs currently sold as dietary ingredients/supplements not only as mere drugs, but as prescription drugs.The reason is very simple even if the argument may be viewed as somewhat of a stretch.
First and foremost, all of the SARMs references in your exceptional article are going to be considered drugs by the FDA and US Attorneys - and this is a correct interpretation which is pretty straightforward. None of the SARMs meet the legal definition of a dietary ingredient under DHSEA 1994 and their appearance in any dietary supplement is an act of misbranding and product adulteration. They are by default, all misbranded drugs. It would be futile to argue this point.
However, FDA and some US Attorneys may successfully argue that SARMs have a reasonable probability of resulting in permanent impairment of a body structure or function in at risk consumers and thus can not be used except under the supervision of a practitioner licensed by law to administer such.
Certainly, by selling SARMs as misbranded drugs under the ruse that they are legal, dietary supplements at 10X the dose, or even higher, studied in any human trials would surely bolster such an assertion by FDA or a US Attorney regarding likelihood of permanent impairment of a body structure or function in at risk consumers anyhow.
While some may view this perspective as a tenuous if not specious (and certainly dose dependent) argument, it's not beyond the pale of something FDA could try to do. In 1951, The Durham-Humphrey Amendment to the FDC became law in the USA. For the edification of you and the PricePlow readers:
"The FDC Act defines a prescription drug at FDC Act § 503(b)(1) as:A drug intended for use by man which –
- (A) because of its toxicity or other potentiality for harmful effect, or the method of its use, or the collateral measures necessary to its use, is not safe for use except under the supervision of a practitioner licensed by law to administer such drug; or
- (B) is limited by an approved application under section 505 to use under the professional supervision of a practitioner licensed by law to administer such drug..
As passed into law in 1951, The FDC Act § 503(b)(1) included three categories of Rx drugs: (1) habit-forming drugs under § 502(d); (2) drugs that are unsafe for use except with a doctor's supervision; and (3) drugs limited to Rx use under an NDA."
[emphases added are Bruce's]
While the thought of FDA viewing SARMS being illegally sold as dietary ingredients/supplements right now as prescription drugs may seem a bit of a stretch, a quick review of FDA's historical enforcement actions regarding aromatase inhibitors being sold as dietary supplements should serve as a prescient and clear warning that this very type of thing surely can happen.
-- Bruce Kneller, Giant Sports Products
...and there you have it.
Current SARM Lawsuits
- IronMagLabs Lawsuit - October 21, 2015
- Revolt Pharma Lawsuit - November 11, 2015
FDA Warning Letters
Note: The following section is originally from a November 2, 2017 blog post titled "SARMs on the Ropes: FDA Finally Issues Warning Letters".

The FDA is on the prowl again, this time they're officially going after three companies who have sold SARMs into the sports nutrition market.
It's been a particularly tumultuous few weeks for the supplement industry with a few recent high-profile arrests and a raid. Riding high on these "victories", the U.S. Food and Drug Administration (FDA) has issued a warning letter the three companies for illegally selling supplements containing SARMs, but this only actually makes sense from a "supplement industry" point of view.
The three companies issued warning letters are:
- Infantry Labs
- IronMag Labs
- Panther Sports Nutrition
The FDA states it has "significant safety concerns" over several of the products marketed as dietary supplements yet they contain selective androgen receptor modulators (SARMs). The FDA contends these products can result in "Life threatening reactions, including liver toxicity" and "also have the potential to increase the risk of heart attack and stroke."[40-42]
SARMs contained in the questionable supplements listed in the warning letters were:
- Ostarine (or MK 2866)
((2S)-3-(4-cyanophenoxy)-N-{4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide
- LGD-4033
4-((R)-2-((R)-2,2,2-trifluoro-1-hydroxyethyl) pyrrolidin-1-yl)-2-trifluoroMethyl) benzonitrile
Citing section 503(b)(1)(A) of the FD&C Act [21 U.S.C. § 353(b)(1)(A)], the FDA states that the SARMs are in fact "prescription drugs" due to their toxicity or possibility for inducing harmful effects with their use.
The compounds have also never been found in nature, which makes them ineligible to be marketed as supplements. But some of the operated in the very gray area of "research chemicals", and were "not for human consumption", although some companies definitely did sell them as supplements earlier on in 2015.
Don't Mess with DASCA
And even if these didn't qualify as prescription drugs, the FDA could also enlist the DEA in making them DASCA-scheduled anabolics, bringing even more heat.
Point being, given the recent Hi-Tech Pharma raid on the andro's, right now is a horrible time to be making anything unnaturally anabolic… which of course means some young cockslinger who's willing to risk jailtime will likely come out of the shadows and take on the new hole in the market.
Brands Speak Out!
We contacted each of the brands that were issued warning letters and received nearly immediate responses:
"We were a little surprised that the FDA sent us a warning letter when a Google search would have shown Super DMZ 4.0 is not on our website nor is it available at any online retailers. Super DMZ 4.0 was a 'one-run' product that we discontinued over two years ago, it was replaced with version 5.0 which does not contain any SARMS.IronMag Labs has not used a SARM in any of our products since 2015, and all of our ingredients and compounds currently used comply with all DSHEA regulations." -- IronMag Labs HQ
One of the other two brands anonymously stated,
"They should probably do a little better research. Nowhere has the bottle ever said 'dietary supplement' and those products have been pulled off the shelves for months."
The FDA states that each company has 15 days to respond to the warning letters with a specific plan to correct the violation and steps to prevent future violations like this from happening down the road. More than likely, these warning letters will be ignored as the brands in question no longer produce/sell these products through their websites.
More Bad News for SARM companies...
This also seems to be game over for those that were fighting Nutrition Distribution in court over the anti-competitive laws. With an FDA warning on their side, we'd think that Nutrition Distribution is almost guaranteed to get a settlement or a court victory, and may be able to re-open any cases they lost.
What are SARMs?
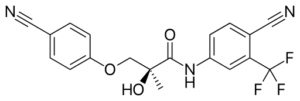
Ostarine Structure: Say hello to Ostarine (MK-2866) one of the most well known and widely used / distributed SARMs.
Anabolic steroids, on the other hand, are more of an "all out assault" that tells impacts all kinds of tissues, including the brain, liver, prostate, and muscles. SARMs are "supposed" to come with fewer side effects due to their more targeted selection of tissues and not the truckload of systemic side effects that can result as a consequence of steroid use. A more apt comparison is that SARMs are like precisely guided strike missiles, while steroids are more like an atomic bomb circa 1945.
Still, SARMs remain a very much untested and proven collection of compounds, but that hasn't stopped several supplement companies from willingly selling them as OTC supplements.
The FDA is slow, but strong

Slow and steady seems to be the FDA's gameplan for relentlessly going after the supplement industry.
It seems the FDA might be a bit backlogged with their endless litigation against Hi-Tech Pharmaceuticals (while, of course, approving deadly suicide-inducing "antidepressants" and creating a national opiate epidemic). Considering these products haven't been in production for over 2 years in some cases, the FDA is proving that doing the crime will eventually catch up to you - but you may be able to make millions in the process.
SARMs are either intellectual property owned by pharmaceutical companies, or unscheduled research chemicals and the long-term use and safety of them is certainly ambiguous, but having a federal agency be this far behind the times with warning letters is an incredibly poor showing on their part.
Right now's a terrible time to be making or selling SARMs, but there's about to be a massive hole in the market. So who's going to enter the ring, and is it worth flirting with DASCA over?
What about Orrin Hatch's SARMs Control Act of 2018?
In 2018, Senator Orrin Hatch introduced the SARMs Control Act of 2018, also known as S. 2742 (115th), attempting to make them illegal. According to GovTrack.us, the bill died in that session of congress,[43] but was reintroduced in 2019 by Chuck Grassley as S. 2895: SARMs Control Act of 2019.[44] Follow GovTrack to stay on top of its updaets.
References
- Dalton, J; "Discovery of Nonsteroidal Androgens"; Biochemical and Biophysical Research Communications; Volume 244, Issue 1, 6 March 1998, Pages 1–4; Retrievedf rom https://www.sciencedirect.com/science/article/pii/S0006291X98982092
- Chen, Jiyun, Juhyun Kim, and James T. Dalton. "Discovery AND Therapeutic Promise OF Selective Androgen Receptor Modulators." Molecular interventions 5.3 (2005): 173–188; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2072877/
- Gao, W; Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs); Drug Discovery Today; Volume 12, Issues 5–6, March 2007, Pages 241–248; Retrieved from https://www.sciencedirect.com/science/article/pii/S1359644607000359
- Cadilla, R; Selective androgen receptor modulators in drug discovery: medicinal chemistry and therapeutic potential.; Curr Top Med Chem. 2006;6(3):245-70; Retrieved from https://pubmed.ncbi.nlm.nih.gov/16515480
- World Anti-Doping Agency; The 2015 Prohibited List; September 20, 2014; Retrieved from https://www.uefa.org/MultimediaFiles/Download/EuroExperience/uefaorg/Anti-doping/02/14/00/26/2140026_DOWNLOAD.pdf
- Hurst, M; Designer drug SRAMS sweeping world sport; The daily Telegraph; February 9, 2012; Retrieved from https://www.dailytelegraph.com.au/sport/designer-drug-srams-sweeping-world-sport/story-fn9dirj0-1226266138458
- Singer, E; Next-Generation Sports Doping; MIT Technology Review; October 26, 2007; Retrieved from https://www.technologyreview.com/news/408954/next-generation-sports-doping/
- Donzé, F; WADA Executive Committee Approves the 2008 Prohibited List; September 23, 2007; Retrieved from https://www.wada-ama.org/en/media/news/2007-09/wada-executive-committee-approves-the-2008-prohibited-list-0
- Carroll, W; "Under the Knife"; Baseball Prospectus; February 5, 2009; Retrieved from https://www.baseballprospectus.com/article.php?articleid=8470
- Ligand Pharmaceuticals; Ligand Initiates Clinical Trial with the Selective Androgen Receptor Modulator LGD-4033, a Potential Treatment of Muscle and Bone Disorders; Retrieved from https://investor.ligand.com/press-releases/detail/45/ligand-initiates-clinical-trial-with-the-selective-androgen
- Ligand Pharmaceuticals; Ligand Presents New Preclinical Data on its Lead SARM Molecule LGD-4033 at the Gerontological Society of America Annual Meeting; November 20, 2009; Retrieved from https://investor.ligand.com/press-releases/detail/150/ligand-presents-new-preclinical-data-on-its-lead-sarm
- Ligand Pharmaceuticals; Ligand Presents First-in-Human Phase I Data on Lead SARM Molecule LGD-4033 at the International Congress of Endocrinology; March 29, 2010; Retrieved from https://investor.ligand.com/press-releases/detail/134/ligand-presents-first-in-human-phase-i-data-on-lead-sarm
- Ligand Pharmaceuticals; Ligand Presents Multi-Dose Phase I Data on Lead SARM Molecule LGD-4033 at the Endocrine Society Annual Meeting; June 6, 2011; Retrieved from https://investor.ligand.com/press-releases/detail/166/ligand-presents-multi-dose-phase-i-data-on-lead-sarm
- Basaria, S; Safety and Tolerability of LGD-4033, a Novel Non-Steroidal Oral Selective Androgen Receptor Modulator (SARM), in Healthy Men; Endocrine Reviews; Vol. 32 (03_MeetingAbstracts): P3-207; Retrieved from https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=9&cad=rja&uact=8&ved=0CFAQFjAIahUKEwiRxtSxkNHHAhXIGpIKHSUfA-A&url=http%3A%2F%2Fphx.corporate-ir.net%2FExternal.File%3Fitem%3DUGFyZW50SUQ9NDI5Njk5fENoaWxkSUQ9NDQ3MDcyfFR5cGU9MQ%3D%3D%26t%3D1&ei=CyLjVdGUA8i1yASlvoyADg&usg=AFQjCNH8sv_qb1P0WO1-VQTZU9ZLNlDrGQ&sig2=DnF09muc2TglU-_Lj0bqQw (backup abstract at https://archive.is/LAt17#selection-331.1-331.125)
- Merck; GTx Presents Phase II Ostarine (MK-2866) Cancer Cachexia Clinical Trial Results at Endocrine Society Annual Meeting: Ostarine Improved Lean Body Mass and Muscle Performance in Patients with Cancer Cachexia; June 11, 2009; Retrieved from https://www.merck.com/licensing/news-and-events/gtx-press-release.html
- McLaughlin, K; Johnson & Johnson; Janssen to Showcase Data from Five Compounds Including Daratumumab and IMBRUVICA at the 2015 American Society of Clinical Oncology (ASCO) Annual Meeting; May 13, 2015; Retrieved from https://www.jnj.com/news/all/Janssen-to-Showcase-Data-from-Five-Compounds-Including-Daratumumab-and-IMBRUVICA-at-the-2015-American-Society-of-Clinical-Oncology-ASCO-Annual-Meeting
- Narayanan, Ramesh et al. "Selective Androgen Receptor Modulators in Preclinical and Clinical Development: Peer-reviewed SARMs from Johnson & Johnson (J&J) and subsidiaries." Nuclear Receptor Signaling 6 (2008): e010; 30 Aug. 2015; Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2602589/figure/fig6/
- GlaxoSmithKline; "GSK Product Development Pipeline"; March 2015; Retrieved from https://www.gsk.com/media/712057/product-pipeline-2015.pdf
- Narayanan, Ramesh et al. "Selective Androgen Receptor Modulators in Preclinical and Clinical Development." Nuclear Receptor Signaling 6 (2008): e010; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2602589/
- GTx Inc; enobosarm (Ostarine; GTx-024); Retrieved from https://www.gtxinc.com/Pipeline/OstarineMK2866.aspx?Sid=4
- He Y, Yin D, Perera M, Kirkovsky L, Stourman N, Li W, Dalton JT, Miller DD. Novel nonsteroidal ligands with high binding affinity and potent functional activity for the androgen receptor; European journal of medicinal chemistry. 2002; 37:619–634; Retrieved from https://www.researchgate.net/profile/Minoli_Perera/publication/11225682_Novel_nonsteroidal_ligands_with_high_binding_affinity_and_potent_functional_activity_for_the_androgen_receptor/links/0046351c49845bf4cc000000.pdf?inViewer=true&disableCoverPage=true&origin=publication_detail
- Marhefka, Craig A. et al. "Design, Synthesis, and Biological Characterization of Metabolically Stable Selective Androgen Receptor Modulators." Journal of medicinal chemistry 47.4 (2004): 993–998; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2040239/
- Dalton, J; US Patent 8853266: "Selective androgen receptor modulators for treating diabetes"; April 13, 2007; https://www.google.com/patents/US8853266
- US Food and Drug Administration; "Dietary Supplement Health and Education Act of 1994"; October 25, 1994; Retrieved from https://www.fda.gov/RegulatoryInformation/Legislation/SignificantAmendmentstotheFDCAct/ucm148003.htm
- United States Congress; "Anabolic Steroid Control Act of 2004"; October 22, 2004; Retrieved from https://www.gpo.gov/fdsys/pkg/BILLS-108s2195enr/pdf/BILLS-108s2195enr.pdf
- Zambrana, I; Food and Drug Administration; Warning Letter to BioGenix USA, LLC; December 11, 2014; Retrieved from https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm434928.htm
- Kloner, R; "Time course of the interaction between tadalafil and nitrates"; Journal of the American College of Cardiology; Volume 42, Issue 10, 19 November 2003, Pages 1855–1860; Retrieved from https://www.sciencedirect.com/science/article/pii/S0735109703012932
- Zhi, L; "Selective androgen receptor modulators (SARMs) and uses thereof"; US Patent and Trademark Office; US Patent #8354446; Retrieved from https://www.google.com/patents/US8354446
- Bigge, C; "Antidiabetic agents"; US Patent and Trademark Office; US Patent #20030171377; Retrieved from https://www.google.com/patents/US20030171377
- Schacht, W; Patent Law and Its Application to the Pharmaceutical Industry: An Examination of the Drug Price Competition and Patent Term Restoration Act of 1984 ("The Hatch-Waxman Act"); Congressional Research Report for Congress; January 10, 2005; Retrieved from https://www.law.umaryland.edu/marshall/crsreports/crsdocuments/rl3075601102005.pdf
- Dalton, James T. et al. "The Selective Androgen Receptor Modulator GTx-024 (enobosarm) Improves Lean Body Mass and Physical Function in Healthy Elderly Men and Postmenopausal Women: Results of a Double-Blind, Placebo-Controlled Phase II Trial." Journal of Cachexia, Sarcopenia and Muscle 2.3 (2011): 153–161; Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3177038/
- Narayanan, Ramesh et al. "Selective Androgen Receptor Modulators in Preclinical and Clinical Development." Nuclear Receptor Signaling 6 (2008); Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2602589/
- GTx, Inc; Form 10-Q Quarterly Report for the Quarterly Period Ended June 30, 2014; United States Securities and Exchange Commission; Retrieved from https://www.sec.gov/Archives/edgar/data/1260990/000110465914056983/a14-14015_110q.htm
- GTx, Inc; Income Statement; Google Finance; Retrieved from https://www.google.com/finance?q=NASDAQ%3AGTXI&fstype=ii
- Geiger, L; "Rat Carcinogenicity with GW501516, a PPAR Delta Agonist"; Society of Toxicology 48th Annual Meeting and ToxExpo; The Toxicologist Supplement to Toxicological Sciences; Oxford University Press; Volume 108, Number 1, March 2009; pp. 189; Retrieved from https://web.archive.org/web/20150504013406/https://www.toxicology.org/AI/PUB/Tox/2009Tox.pdf
- Sahebkar, A; "New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease."; Expert Opin Pharmacother. 2014 Mar;15(4):493-503; Retrieved from https://pubmed.ncbi.nlm.nih.gov/24428677
- World Anti-Doping Agency; "WADA issues alert on GW501516"; March 21, 2013; Retrieved from https://web.archive.org/web/20130602050612/https://playtrue.wada-ama.org/news/wada-issues-alert-on-gw501516/
- New Scientist; Anti-doping Agency Warns Athletes of Black Market Drug; March 26, 2013; Retrieved from https://www.newscientist.com/article/mg21729103-400-anti-doping-agency-warns-athletes-of-black-market-drug/
- Rossen, J; "A New Class of Unapproved Supplements Has Mass Appeal"; Maxim Magazine; April 14, 2015; Retrieved from https://www.maxim.com/maxim-man/fitness/article/new-class-unapproved-supplements-has-mass-appeal
- https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2017/ucm582685.htm
- https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2017/ucm582464.htm
- https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2017/ucm581630.htm
- https://www.govtrack.us/congress/bills/115/s2742
- https://www.govtrack.us/congress/bills/116/s2895
SARM News on PricePlow
-
Mar 19, 2025 
YouTube Video
Olympic Level Supplement Testing: Andy Holmes Explains LGC's Informed Sport | Episode 162 -
Jun 01, 2024 
YouTube Video
Ryan Garcia (Boxer) Blames NutraBio for Failed Drug Test?! (Ostarine) -
Mar 11, 2024 
YouTube Video
Michael Bischoff of Glaxon INDICTED and Prosecuted (SARMs Charge) -
Nov 20, 2022 
TikTok Video
PSA -
Nov 20, 2019 
Blog Post
IronMagLabs Lawsuit Dismissal REVERSED, Case Re-Opened (2019 Update: DiMaggio Guilty in Other Case)
IronMagLabs has been sued over their use of SARMs in muscle-building products by a competitor (Nutrition Distribution) for unfair competition! -
May 14, 2019 
YouTube Video
TALK OF THE TOWN: BEN'S VIEWS ON MARK BELL'S SARMAGEDDON -
Feb 17, 2019 
Blog Post
Muscle Building Supplements: The Next Generation (2019)
Muscle Building Supplements: The 2016 Guide. We all know about creatine, protein, and amino acids... but what are the NEXT generation muscle builders? -
Apr 12, 2018 
YouTube Video
Enhanced Athlete Lawsuit UPDATE: Tony Huge to be Deposed (April 2018) -
Mar 26, 2018 
Blog Post
The Lanham Act: How POM vs Coke Enabled Supplement Lawsuits
What's POM Wonderful and Coca-Cola got to do with supplements? The fact that their lawsuit led to the opening of inter-industry Lanham Act LAWSUITS! -
Jan 03, 2018 
Blog Post
The Supplement Industry 2017 Year in Review: The 10 Biggest Stories
2017 may be gone but it's not forgotten. Check out all of the biggest sports nutrition supplement news stories from 2017 as we recap the year 2017. -
Nov 10, 2015 
Blog Post
RICO Case Filed Against IronMagLabs by Hi-Tech Pharma
IronMagLabs has been sued once again, this time by Hi-Tech Pharmaceuticals for patent infringement, unfair competition, and a RICO case is brought! -
Oct 12, 2015 
Blog Post
Will Grier DOPING Scandal: Suspended for SARM Use at Florida
Will Grier (Florida Gators QB) has been suspended for Performance Enhancing Drug use. He tested positive for Ligandrol. We clarify the situation.
Sign up for future SARM news!
Click the button below to sign up for future SARM news, deals, coupons, and reviews!
SARM Reviews & Videos
-
Mar 19, 2025Olympic Level Supplement Testing: Andy Holmes Explains LGC's Informed Sport | Episode 162
-
Jun 01, 2024Ryan Garcia (Boxer) Blames NutraBio for Failed Drug Test?! (Ostarine)
-
Mar 11, 2024Michael Bischoff of Glaxon INDICTED and Prosecuted (SARMs Charge)
-
Nov 20, 2022PSA
-
May 14, 2019TALK OF THE TOWN: BEN'S VIEWS ON MARK BELL'S SARMAGEDDON
-
Apr 12, 2018Enhanced Athlete Lawsuit UPDATE: Tony Huge to be Deposed (April 2018)
Subscribe for more SARM news and alerts!
Subscribe to PricePlow on YouTube, follow PricePlow on Instagram or click the button below to sign up for our latest SARM news and reviews!